Data Manipulation with dplyr
Data analysis involves a large amount of janitor work – munging and cleaning data to facilitate downstream data analysis. This lesson demonstrates techniques for advanced data manipulation and analysis with the split-apply-combine strategy. We will use the dplyr package in R to effectively manipulate and conditionally compute summary statistics over subsets of a “big” dataset containing many observations.
This lesson assumes a basic familiarity with R and data frames.
Recommended reading: Review the Introduction (10.1) and Tibbles vs. data.frame (10.3) sections of the R for Data Science book. We will initially be using the read_* functions from the readr package. These functions load data into a tibble instead of R’s traditional data.frame. Tibbles are data frames, but they tweak some older behaviors to make life a little easier. These sections explain the few key small differences between traditional data.frames and tibbles.
Review
Our data
We’re going to use the yeast gene expression dataset described on the data frames lesson. This is a cleaned up version of a gene expression dataset from Brauer et al. Coordination of Growth Rate, Cell Cycle, Stress Response, and Metabolic Activity in Yeast (2008) Mol Biol Cell 19:352-367. This data is from a gene expression microarray, and in this paper the authors are examining the relationship between growth rate and gene expression in yeast cultures limited by one of six different nutrients (glucose, leucine, ammonium, sulfate, phosphate, uracil). If you give yeast a rich media loaded with nutrients except restrict the supply of a single nutrient, you can control the growth rate to any rate you choose. By starving yeast of specific nutrients you can find genes that:
- Raise or lower their expression in response to growth rate. Growth-rate dependent expression patterns can tell us a lot about cell cycle control, and how the cell responds to stress. The authors found that expression of >25% of all yeast genes is linearly correlated with growth rate, independent of the limiting nutrient. They also found that the subset of negatively growth-correlated genes is enriched for peroxisomal functions, and positively correlated genes mainly encode ribosomal functions.
- Respond differently when different nutrients are being limited. If you see particular genes that respond very differently when a nutrient is sharply restricted, these genes might be involved in the transport or metabolism of that specific nutrient.
You can download the cleaned up version of the data at the link above. The file is called brauer2007_tidy.csv. Later on we’ll actually start with the original raw data (minimally processed) and manipulate it so that we can make it more amenable for analysis.
Reading in data
We need to load both the dplyr and readr packages for efficiently reading in and displaying this data. We’re also going to use many other functions from the dplyr package. Make sure you have these packages installed as described on the setup page.
# Load packages
library(readr)
library(dplyr)
# Read in data
ydat <- read_csv(file="data/brauer2007_tidy.csv")
# Display the data
ydat
# Optionally, bring up the data in a viewer window
# View(ydat)## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsThe dplyr package
The dplyr package is a relatively new R package that makes data manipulation fast and easy. It imports functionality from another package called magrittr that allows you to chain commands together into a pipeline that will completely change the way you write R code such that you’re writing code the way you’re thinking about the problem.
When you read in data with the readr package (read_csv()) and you had the dplyr package loaded already, the data frame takes on this “special” class of data frames called a tbl (pronounced “tibble”), which you can see with class(ydat). If you have other “regular” data frames in your workspace, the as_tibble() function will convert it into the special dplyr tbl that displays nicely (e.g.: iris <- as_tibble(iris)). You don’t have to turn all your data frame objects into tibbles, but it does make working with large datasets a bit easier.
You can read more about tibbles in Tibbles chapter in R for Data Science or in the tibbles vignette. They keep most of the features of data frames, and drop the features that used to be convenient but are now frustrating (i.e. converting character vectors to factors). You can read more about the differences between data frames and tibbles in this section of the tibbles vignette, but the major convenience for us concerns printing (aka displaying) a tibble to the screen. When you print (i.e., display) a tibble, it only shows the first 10 rows and all the columns that fit on one screen. It also prints an abbreviated description of the column type. You can control the default appearance with options:
options(tibble.print_max = n, tibble.print_min = m): if there are more than n rows, print only the first m rows. Useoptions(tibble.print_max = Inf)to always show all rows.options(tibble.width = Inf)will always print all columns, regardless of the width of the screen.
dplyr verbs
The dplyr package gives you a handful of useful verbs for managing data. On their own they don’t do anything that base R can’t do. Here are some of the single-table verbs we’ll be working with in this lesson (single-table meaning that they only work on a single table – contrast that to two-table verbs used for joining data together, which we’ll cover in a later lesson).
filter()select()mutate()arrange()summarize()group_by()
They all take a data frame or tibble as their input for the first argument, and they all return a data frame or tibble as output.
filter()
If you want to filter rows of the data where some condition is true, use the filter() function.
- The first argument is the data frame you want to filter, e.g.
filter(mydata, .... - The second argument is a condition you must satisfy, e.g.
filter(ydat, symbol == "LEU1"). If you want to satisfy all of multiple conditions, you can use the “and” operator,&. The “or” operator|(the pipe character, usually shift-backslash) will return a subset that meet any of the conditions.
==: Equal to!=: Not equal to>,>=: Greater than, greater than or equal to<,<=: Less than, less than or equal to
Let’s try it out. For this to work you have to have already loaded the dplyr package. Let’s take a look at LEU1, a gene involved in leucine synthesis.
# First, make sure you've loaded the dplyr package
library(dplyr)
# Look at a single gene involved in leucine synthesis pathway
filter(ydat, symbol == "LEU1")## # A tibble: 36 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Glucose 0.05 -1.12 leucine … 3-isopropyl…
## 2 LEU1 YGL009C Glucose 0.1 -0.77 leucine … 3-isopropyl…
## 3 LEU1 YGL009C Glucose 0.15 -0.67 leucine … 3-isopropyl…
## 4 LEU1 YGL009C Glucose 0.2 -0.59 leucine … 3-isopropyl…
## 5 LEU1 YGL009C Glucose 0.25 -0.2 leucine … 3-isopropyl…
## 6 LEU1 YGL009C Glucose 0.3 0.03 leucine … 3-isopropyl…
## 7 LEU1 YGL009C Ammonia 0.05 -0.76 leucine … 3-isopropyl…
## 8 LEU1 YGL009C Ammonia 0.1 -1.17 leucine … 3-isopropyl…
## 9 LEU1 YGL009C Ammonia 0.15 -1.2 leucine … 3-isopropyl…
## 10 LEU1 YGL009C Ammonia 0.2 -1.02 leucine … 3-isopropyl…
## # ... with 26 more rows# Optionally, bring that result up in a View window
# View(filter(ydat, symbol == "LEU1"))
# Look at multiple genes
filter(ydat, symbol=="LEU1" | symbol=="ADH2")## # A tibble: 72 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Glucose 0.05 -1.12 leucine … 3-isopropyl…
## 2 ADH2 YMR303C Glucose 0.05 6.28 fermenta… alcohol deh…
## 3 LEU1 YGL009C Glucose 0.1 -0.77 leucine … 3-isopropyl…
## 4 ADH2 YMR303C Glucose 0.1 5.81 fermenta… alcohol deh…
## 5 LEU1 YGL009C Glucose 0.15 -0.67 leucine … 3-isopropyl…
## 6 ADH2 YMR303C Glucose 0.15 5.64 fermenta… alcohol deh…
## 7 LEU1 YGL009C Glucose 0.2 -0.59 leucine … 3-isopropyl…
## 8 ADH2 YMR303C Glucose 0.2 5.1 fermenta… alcohol deh…
## 9 LEU1 YGL009C Glucose 0.25 -0.2 leucine … 3-isopropyl…
## 10 ADH2 YMR303C Glucose 0.25 1.89 fermenta… alcohol deh…
## # ... with 62 more rows# Look at LEU1 expression at a low growth rate due to nutrient depletion
# Notice how LEU1 is highly upregulated when leucine is depleted!
filter(ydat, symbol=="LEU1" & rate==.05)## # A tibble: 6 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Glucose 0.05 -1.12 leucine … 3-isopropyl…
## 2 LEU1 YGL009C Ammonia 0.05 -0.76 leucine … 3-isopropyl…
## 3 LEU1 YGL009C Phosphate 0.05 -0.81 leucine … 3-isopropyl…
## 4 LEU1 YGL009C Sulfate 0.05 -1.57 leucine … 3-isopropyl…
## 5 LEU1 YGL009C Leucine 0.05 3.84 leucine … 3-isopropyl…
## 6 LEU1 YGL009C Uracil 0.05 -2.07 leucine … 3-isopropyl…# But expression goes back down when the growth/nutrient restriction is relaxed
filter(ydat, symbol=="LEU1" & rate==.3)## # A tibble: 6 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Glucose 0.3 0.03 leucine … 3-isopropyl…
## 2 LEU1 YGL009C Ammonia 0.3 -0.22 leucine … 3-isopropyl…
## 3 LEU1 YGL009C Phosphate 0.3 -0.07 leucine … 3-isopropyl…
## 4 LEU1 YGL009C Sulfate 0.3 -0.76 leucine … 3-isopropyl…
## 5 LEU1 YGL009C Leucine 0.3 0.87 leucine … 3-isopropyl…
## 6 LEU1 YGL009C Uracil 0.3 -0.16 leucine … 3-isopropyl…# Show only stats for LEU1 and Leucine depletion.
# LEU1 expression starts off high and drops
filter(ydat, symbol=="LEU1" & nutrient=="Leucine")## # A tibble: 6 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Leucine 0.05 3.84 leucine … 3-isopropylm…
## 2 LEU1 YGL009C Leucine 0.1 3.36 leucine … 3-isopropylm…
## 3 LEU1 YGL009C Leucine 0.15 3.24 leucine … 3-isopropylm…
## 4 LEU1 YGL009C Leucine 0.2 2.84 leucine … 3-isopropylm…
## 5 LEU1 YGL009C Leucine 0.25 2.04 leucine … 3-isopropylm…
## 6 LEU1 YGL009C Leucine 0.3 0.87 leucine … 3-isopropylm…# What about LEU1 expression with other nutrients being depleted?
filter(ydat, symbol=="LEU1" & nutrient=="Glucose")## # A tibble: 6 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 LEU1 YGL009C Glucose 0.05 -1.12 leucine … 3-isopropylm…
## 2 LEU1 YGL009C Glucose 0.1 -0.77 leucine … 3-isopropylm…
## 3 LEU1 YGL009C Glucose 0.15 -0.67 leucine … 3-isopropylm…
## 4 LEU1 YGL009C Glucose 0.2 -0.59 leucine … 3-isopropylm…
## 5 LEU1 YGL009C Glucose 0.25 -0.2 leucine … 3-isopropylm…
## 6 LEU1 YGL009C Glucose 0.3 0.03 leucine … 3-isopropylm…Let’s look at this graphically. Don’t worry about what these commands are doing just yet - we’ll cover that later on when we talk about ggplot2. Here’s I’m taking the filtered dataset containing just expression estimates for LEU1 where I have 36 rows (one for each of 6 nutrients \(\times\) 6 growth rates), and I’m piping that dataset to the plotting function, where I’m plotting rate on the x-axis, expression on the y-axis, mapping the value of nutrient to the color, and using a line plot to display the data.
library(ggplot2)
filter(ydat, symbol=="LEU1") %>%
ggplot(aes(rate, expression, colour=nutrient)) + geom_line(lwd=1.5)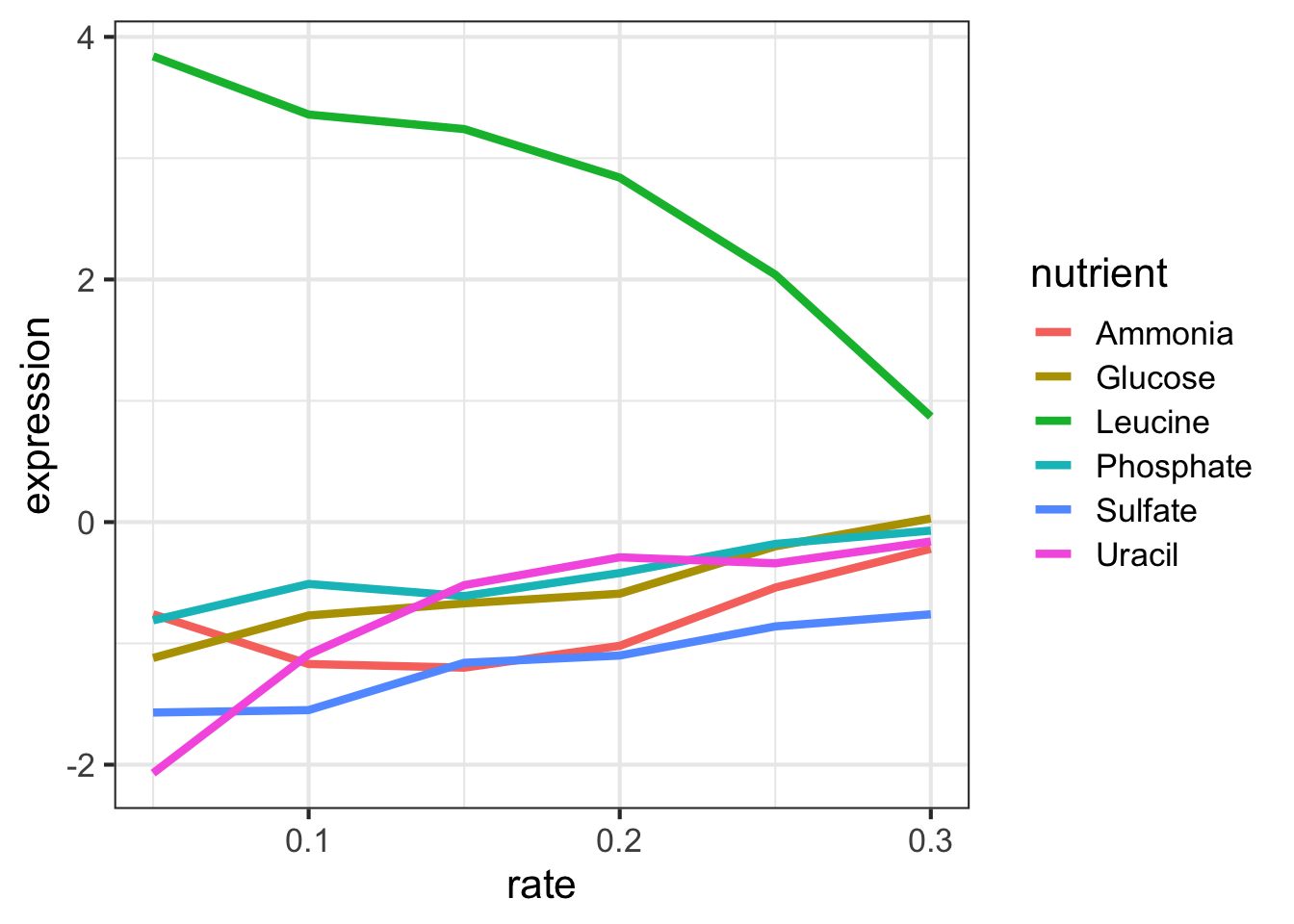
Look closely at that! LEU1 is highly expressed when starved of leucine because the cell has to synthesize its own! And as the amount of leucine in the environment (the growth rate) increases, the cell can worry less about synthesizing leucine, so LEU1 expression goes back down. Consequently the cell can devote more energy into other functions, and we see other genes’ expression very slightly raising.
EXERCISE 1
- Display the data where the gene ontology biological process (the
bpvariable) is “leucine biosynthesis” (case-sensitive) and the limiting nutrient was Leucine. (Answer should return a 24-by-7 data frame – 4 genes \(\times\) 6 growth rates). - Gene/rate combinations had high expression (in the top 1% of expressed genes)? Hint: see
?quantileand tryquantile(ydat$expression, probs=.99)to see the expression value which is higher than 99% of all the data, thenfilter()based on that. Try wrapping your answer with aView()function so you can see the whole thing. What does it look like those genes are doing? Answer should return a 1971-by-7 data frame.
Aside: Writing Data to File
What we’ve done up to this point is read in data from a file (read_csv(...)), and assigning that to an object in our workspace (ydat <- ...). When we run operations like filter() on our data, consider two things:
- The
ydatobject in our workspace is not being modified directly. That is, we canfilter(ydat, ...), and a result is returned to the screen, butydatremains the same. This effect is similar to what we demonstrated in our first session.
# Assign the value '50' to the weight object.
weight <- 50
# Print out weight to the screen (50)
weight
# What's the value of weight plus 10?
weight + 10
# Weight is still 50
weight
# Weight is only modified if we *reassign* weight to the modified value
weight <- weight+10
# Weight is now 60
weight- More importantly, the data file on disk (
data/brauer2007_tidy.csv) is never modified. No matter what we do to ydat, the file is never modified. If we want to save the result of an operation to a file on disk, we can assign the result of an operation to an object, andwrite_csvthat object to disk. See the help for?write_csv(note,write_csv()with an underscore is part of the readr package – not to be confused with the built-inwrite.csv()function).
# What's the result of this filter operation?
filter(ydat, nutrient=="Leucine" & bp=="leucine biosynthesis")
# Assign the result to a new object
leudat <- filter(ydat, nutrient=="Leucine" & bp=="leucine biosynthesis")
# Write that out to disk
write_csv(leudat, "leucinedata.csv")Note that this is different than saving your entire workspace to an Rdata file, which would contain all the objects we’ve created (weight, ydat, leudat, etc).
select()
The filter() function allows you to return only certain rows matching a condition. The select() function returns only certain columns. The first argument is the data, and subsequent arguments are the columns you want.
# Select just the symbol and systematic_name
select(ydat, symbol, systematic_name)## # A tibble: 198,430 x 2
## symbol systematic_name
## <chr> <chr>
## 1 SFB2 YNL049C
## 2 <NA> YNL095C
## 3 QRI7 YDL104C
## 4 CFT2 YLR115W
## 5 SSO2 YMR183C
## 6 PSP2 YML017W
## 7 RIB2 YOL066C
## 8 VMA13 YPR036W
## 9 EDC3 YEL015W
## 10 VPS5 YOR069W
## # ... with 198,420 more rows# Alternatively, just remove columns. Remove the bp and mf columns.
select(ydat, -bp, -mf)## # A tibble: 198,430 x 5
## symbol systematic_name nutrient rate expression
## <chr> <chr> <chr> <dbl> <dbl>
## 1 SFB2 YNL049C Glucose 0.05 -0.24
## 2 <NA> YNL095C Glucose 0.05 0.28
## 3 QRI7 YDL104C Glucose 0.05 -0.02
## 4 CFT2 YLR115W Glucose 0.05 -0.33
## 5 SSO2 YMR183C Glucose 0.05 0.05
## 6 PSP2 YML017W Glucose 0.05 -0.69
## 7 RIB2 YOL066C Glucose 0.05 -0.55
## 8 VMA13 YPR036W Glucose 0.05 -0.75
## 9 EDC3 YEL015W Glucose 0.05 -0.24
## 10 VPS5 YOR069W Glucose 0.05 -0.16
## # ... with 198,420 more rows# Notice that the original data doesn't change!
ydat## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsNotice above how the original data doesn’t change. We’re selecting out only certain columns of interest and throwing away columns we don’t care about. If we wanted to keep this data, we would need to reassign the result of the select() operation to a new object. Let’s make a new object called nogo that does not contain the GO annotations. Notice again how the original data is unchanged.
# create a new dataset without the go annotations.
nogo <- select(ydat, -bp, -mf)
nogo## # A tibble: 198,430 x 5
## symbol systematic_name nutrient rate expression
## <chr> <chr> <chr> <dbl> <dbl>
## 1 SFB2 YNL049C Glucose 0.05 -0.24
## 2 <NA> YNL095C Glucose 0.05 0.28
## 3 QRI7 YDL104C Glucose 0.05 -0.02
## 4 CFT2 YLR115W Glucose 0.05 -0.33
## 5 SSO2 YMR183C Glucose 0.05 0.05
## 6 PSP2 YML017W Glucose 0.05 -0.69
## 7 RIB2 YOL066C Glucose 0.05 -0.55
## 8 VMA13 YPR036W Glucose 0.05 -0.75
## 9 EDC3 YEL015W Glucose 0.05 -0.24
## 10 VPS5 YOR069W Glucose 0.05 -0.16
## # ... with 198,420 more rows# we could filter this new dataset
filter(nogo, symbol=="LEU1" & rate==.05)## # A tibble: 6 x 5
## symbol systematic_name nutrient rate expression
## <chr> <chr> <chr> <dbl> <dbl>
## 1 LEU1 YGL009C Glucose 0.05 -1.12
## 2 LEU1 YGL009C Ammonia 0.05 -0.76
## 3 LEU1 YGL009C Phosphate 0.05 -0.81
## 4 LEU1 YGL009C Sulfate 0.05 -1.57
## 5 LEU1 YGL009C Leucine 0.05 3.84
## 6 LEU1 YGL009C Uracil 0.05 -2.07# Notice how the original data is unchanged - still have all 7 columns
ydat## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsmutate()
The mutate() function adds new columns to the data. Remember, it doesn’t actually modify the data frame you’re operating on, and the result is transient unless you assign it to a new object or reassign it back to itself (generally, not always a good practice).
The expression level reported here is the \(log_2\) of the sample signal divided by the signal in the reference channel, where the reference RNA for all samples was taken from the glucose-limited chemostat grown at a dilution rate of 0.25 \(h^{-1}\). Let’s mutate this data to add a new variable called “signal” that’s the actual raw signal ratio instead of the log-transformed signal.
mutate(nogo, signal=2^expression)Mutate has a nice little feature too in that it’s “lazy.” You can mutate and add one variable, then continue mutating to add more variables based on that variable. Let’s make another column that’s the square root of the signal ratio.
mutate(nogo, signal=2^expression, sigsr=sqrt(signal))## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression signal sigsr
## <chr> <chr> <chr> <dbl> <dbl> <dbl> <dbl>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 0.847 0.920
## 2 <NA> YNL095C Glucose 0.05 0.28 1.21 1.10
## 3 QRI7 YDL104C Glucose 0.05 -0.02 0.986 0.993
## 4 CFT2 YLR115W Glucose 0.05 -0.33 0.796 0.892
## 5 SSO2 YMR183C Glucose 0.05 0.05 1.04 1.02
## 6 PSP2 YML017W Glucose 0.05 -0.69 0.620 0.787
## 7 RIB2 YOL066C Glucose 0.05 -0.55 0.683 0.826
## 8 VMA13 YPR036W Glucose 0.05 -0.75 0.595 0.771
## 9 EDC3 YEL015W Glucose 0.05 -0.24 0.847 0.920
## 10 VPS5 YOR069W Glucose 0.05 -0.16 0.895 0.946
## # ... with 198,420 more rowsAgain, don’t worry about the code here to make the plot – we’ll learn about this later. Why do you think we log-transform the data prior to analysis?
library(tidyr)
mutate(nogo, signal=2^expression, sigsr=sqrt(signal)) %>%
gather(unit, value, expression:sigsr) %>%
ggplot(aes(value)) + geom_histogram(bins=100) + facet_wrap(~unit, scales="free")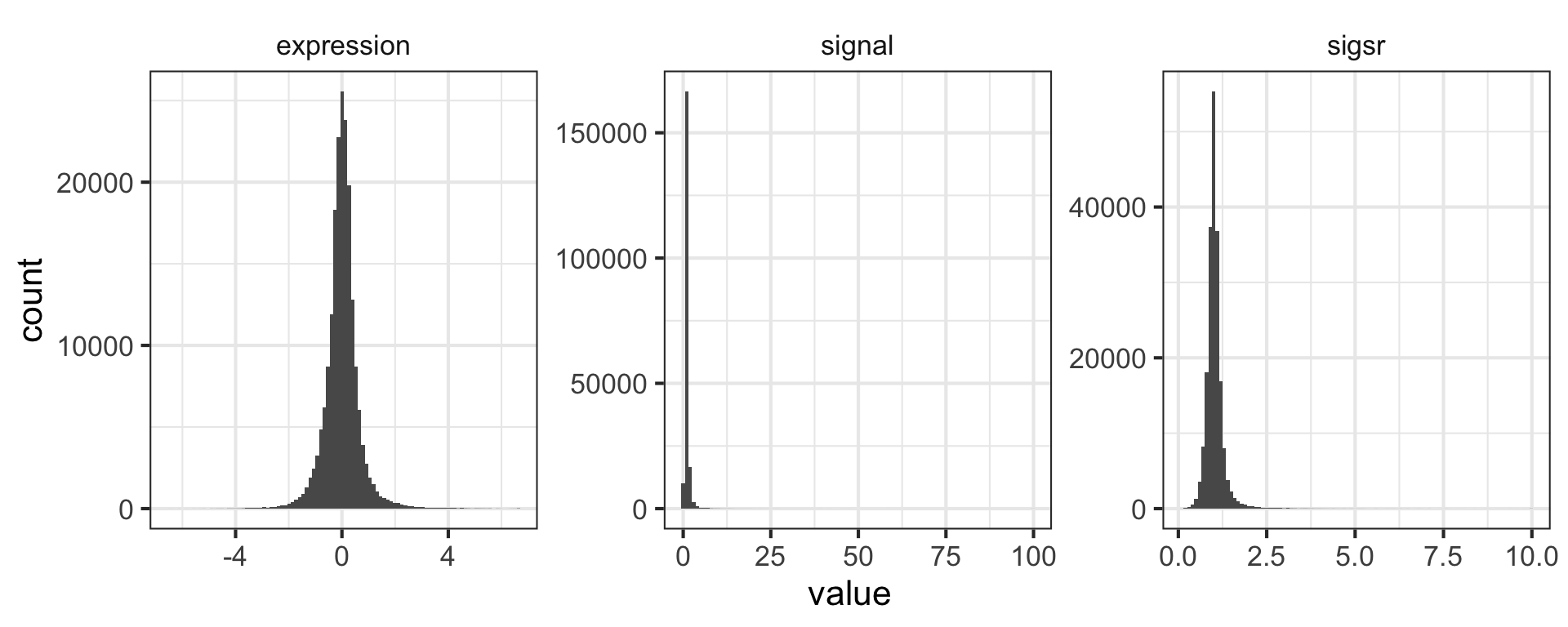
arrange()
The arrange() function does what it sounds like. It takes a data frame or tbl and arranges (or sorts) by column(s) of interest. The first argument is the data, and subsequent arguments are columns to sort on. Use the desc() function to arrange by descending.
# arrange by gene symbol
arrange(ydat, symbol)## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 AAC1 YMR056C Glucose 0.05 1.5 aerobic r… ATP:ADP an…
## 2 AAC1 YMR056C Glucose 0.1 1.54 aerobic r… ATP:ADP an…
## 3 AAC1 YMR056C Glucose 0.15 1.16 aerobic r… ATP:ADP an…
## 4 AAC1 YMR056C Glucose 0.2 1.04 aerobic r… ATP:ADP an…
## 5 AAC1 YMR056C Glucose 0.25 0.84 aerobic r… ATP:ADP an…
## 6 AAC1 YMR056C Glucose 0.3 0.01 aerobic r… ATP:ADP an…
## 7 AAC1 YMR056C Ammonia 0.05 0.8 aerobic r… ATP:ADP an…
## 8 AAC1 YMR056C Ammonia 0.1 1.47 aerobic r… ATP:ADP an…
## 9 AAC1 YMR056C Ammonia 0.15 0.97 aerobic r… ATP:ADP an…
## 10 AAC1 YMR056C Ammonia 0.2 0.76 aerobic r… ATP:ADP an…
## # ... with 198,420 more rows# arrange by expression (default: increasing)
arrange(ydat, expression)## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SUL1 YBR294W Phosphate 0.05 -6.5 sulfate … sulfate tr…
## 2 SUL1 YBR294W Phosphate 0.1 -6.34 sulfate … sulfate tr…
## 3 ADH2 YMR303C Phosphate 0.1 -6.15 fermenta… alcohol de…
## 4 ADH2 YMR303C Phosphate 0.3 -6.04 fermenta… alcohol de…
## 5 ADH2 YMR303C Phosphate 0.25 -5.89 fermenta… alcohol de…
## 6 SUL1 YBR294W Uracil 0.05 -5.55 sulfate … sulfate tr…
## 7 SFC1 YJR095W Phosphate 0.2 -5.52 fumarate… succinate:…
## 8 JEN1 YKL217W Phosphate 0.3 -5.44 lactate … lactate tr…
## 9 MHT1 YLL062C Phosphate 0.05 -5.36 sulfur a… homocystei…
## 10 SFC1 YJR095W Phosphate 0.25 -5.35 fumarate… succinate:…
## # ... with 198,420 more rows# arrange by decreasing expression
arrange(ydat, desc(expression))## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 GAP1 YKR039W Ammonia 0.05 6.64 amino aci… L-proline…
## 2 DAL5 YJR152W Ammonia 0.05 6.64 allantoat… allantoat…
## 3 GAP1 YKR039W Ammonia 0.1 6.64 amino aci… L-proline…
## 4 DAL5 YJR152W Ammonia 0.1 6.64 allantoat… allantoat…
## 5 DAL5 YJR152W Ammonia 0.15 6.64 allantoat… allantoat…
## 6 DAL5 YJR152W Ammonia 0.2 6.64 allantoat… allantoat…
## 7 DAL5 YJR152W Ammonia 0.25 6.64 allantoat… allantoat…
## 8 DAL5 YJR152W Ammonia 0.3 6.64 allantoat… allantoat…
## 9 GIT1 YCR098C Phosphate 0.05 6.64 glyceroph… glyceroph…
## 10 PHM6 YDR281C Phosphate 0.05 6.64 biologica… molecular…
## # ... with 198,420 more rowsEXERCISE 2
- First, re-run the command you used above to filter the data for genes involved in the “leucine biosynthesis” biological process and where the limiting nutrient is Leucine.
- Wrap this entire filtered result with a call to
arrange()where you’ll arrange the result of #1 by the gene symbol. - Wrap this entire result in a
View()statement so you can see the entire result.
summarize()
The summarize() function summarizes multiple values to a single value. On its own the summarize() function doesn’t seem to be all that useful. The dplyr package provides a few convenience functions called n() and n_distinct() that tell you the number of observations or the number of distinct values of a particular variable.
Notice that summarize takes a data frame and returns a data frame. In this case it’s a 1x1 data frame with a single row and a single column. The name of the column, by default is whatever the expression was used to summarize the data. This usually isn’t pretty, and if we wanted to work with this resulting data frame later on, we’d want to name that returned value something easier to deal with.
# Get the mean expression for all genes
summarize(ydat, mean(expression))## # A tibble: 1 x 1
## `mean(expression)`
## <dbl>
## 1 0.00337# Use a more friendly name, e.g., meanexp, or whatever you want to call it.
summarize(ydat, meanexp=mean(expression))## # A tibble: 1 x 1
## meanexp
## <dbl>
## 1 0.00337# Measure the correlation between rate and expression
summarize(ydat, r=cor(rate, expression))## # A tibble: 1 x 1
## r
## <dbl>
## 1 -0.0220# Get the number of observations
summarize(ydat, n())## # A tibble: 1 x 1
## `n()`
## <int>
## 1 198430# The number of distinct gene symbols in the data
summarize(ydat, n_distinct(symbol))## # A tibble: 1 x 1
## `n_distinct(symbol)`
## <int>
## 1 4211group_by()
We saw that summarize() isn’t that useful on its own. Neither is group_by() All this does is takes an existing data frame and coverts it into a grouped data frame where operations are performed by group.
ydat## # A tibble: 198,430 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsgroup_by(ydat, nutrient)## # A tibble: 198,430 x 7
## # Groups: nutrient [6]
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsgroup_by(ydat, nutrient, rate)## # A tibble: 198,430 x 7
## # Groups: nutrient, rate [36]
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Glucose 0.05 -0.24 ER to Gol… molecular …
## 2 <NA> YNL095C Glucose 0.05 0.28 biologica… molecular …
## 3 QRI7 YDL104C Glucose 0.05 -0.02 proteolys… metalloend…
## 4 CFT2 YLR115W Glucose 0.05 -0.33 mRNA poly… RNA binding
## 5 SSO2 YMR183C Glucose 0.05 0.05 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Glucose 0.05 -0.69 biologica… molecular …
## 7 RIB2 YOL066C Glucose 0.05 -0.55 riboflavi… pseudourid…
## 8 VMA13 YPR036W Glucose 0.05 -0.75 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Glucose 0.05 -0.24 deadenyly… molecular …
## 10 VPS5 YOR069W Glucose 0.05 -0.16 protein r… protein tr…
## # ... with 198,420 more rowsThe real power comes in where group_by() and summarize() are used together. First, write the group_by() statement. Then wrap the result of that with a call to summarize().
# Get the mean expression for each gene
# group_by(ydat, symbol)
summarize(group_by(ydat, symbol), meanexp=mean(expression))## # A tibble: 4,211 x 2
## symbol meanexp
## <chr> <dbl>
## 1 AAC1 0.529
## 2 AAC3 -0.216
## 3 AAD10 0.438
## 4 AAD14 -0.0717
## 5 AAD16 0.242
## 6 AAD4 -0.792
## 7 AAD6 0.290
## 8 AAH1 0.0461
## 9 AAP1 -0.00361
## 10 AAP1' -0.421
## # ... with 4,201 more rows# Get the correlation between rate and expression for each nutrient
# group_by(ydat, nutrient)
summarize(group_by(ydat, nutrient), r=cor(rate, expression))## # A tibble: 6 x 2
## nutrient r
## <chr> <dbl>
## 1 Ammonia -0.0175
## 2 Glucose -0.0112
## 3 Leucine -0.0384
## 4 Phosphate -0.0194
## 5 Sulfate -0.0166
## 6 Uracil -0.0353The pipe: %>%
How %>% works
This is where things get awesome. The dplyr package imports functionality from the magrittr package that lets you pipe the output of one function to the input of another, so you can avoid nesting functions. It looks like this: %>%. You don’t have to load the magrittr package to use it since dplyr imports its functionality when you load the dplyr package.
Here’s the simplest way to use it. Remember the tail() function. It expects a data frame as input, and the next argument is the number of lines to print. These two commands are identical:
tail(ydat, 5)## # A tibble: 5 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 KRE1 YNL322C Uracil 0.3 0.28 cell wall o… structura…
## 2 MTL1 YGR023W Uracil 0.3 0.27 cell wall o… molecular…
## 3 KRE9 YJL174W Uracil 0.3 0.43 cell wall o… molecular…
## 4 UTH1 YKR042W Uracil 0.3 0.19 mitochondri… molecular…
## 5 <NA> YOL111C Uracil 0.3 0.04 biological … molecular…ydat %>% tail(5)## # A tibble: 5 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 KRE1 YNL322C Uracil 0.3 0.28 cell wall o… structura…
## 2 MTL1 YGR023W Uracil 0.3 0.27 cell wall o… molecular…
## 3 KRE9 YJL174W Uracil 0.3 0.43 cell wall o… molecular…
## 4 UTH1 YKR042W Uracil 0.3 0.19 mitochondri… molecular…
## 5 <NA> YOL111C Uracil 0.3 0.04 biological … molecular…Let’s use one of the dplyr verbs.
filter(ydat, nutrient=="Leucine")## # A tibble: 33,178 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Leucine 0.05 0.18 ER to Gol… molecular …
## 2 <NA> YNL095C Leucine 0.05 0.16 biologica… molecular …
## 3 QRI7 YDL104C Leucine 0.05 -0.3 proteolys… metalloend…
## 4 CFT2 YLR115W Leucine 0.05 -0.27 mRNA poly… RNA binding
## 5 SSO2 YMR183C Leucine 0.05 -0.59 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Leucine 0.05 -0.17 biologica… molecular …
## 7 RIB2 YOL066C Leucine 0.05 -0.02 riboflavi… pseudourid…
## 8 VMA13 YPR036W Leucine 0.05 -0.11 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Leucine 0.05 0.12 deadenyly… molecular …
## 10 VPS5 YOR069W Leucine 0.05 -0.2 protein r… protein tr…
## # ... with 33,168 more rowsydat %>% filter(nutrient=="Leucine")## # A tibble: 33,178 x 7
## symbol systematic_name nutrient rate expression bp mf
## <chr> <chr> <chr> <dbl> <dbl> <chr> <chr>
## 1 SFB2 YNL049C Leucine 0.05 0.18 ER to Gol… molecular …
## 2 <NA> YNL095C Leucine 0.05 0.16 biologica… molecular …
## 3 QRI7 YDL104C Leucine 0.05 -0.3 proteolys… metalloend…
## 4 CFT2 YLR115W Leucine 0.05 -0.27 mRNA poly… RNA binding
## 5 SSO2 YMR183C Leucine 0.05 -0.59 vesicle f… t-SNARE ac…
## 6 PSP2 YML017W Leucine 0.05 -0.17 biologica… molecular …
## 7 RIB2 YOL066C Leucine 0.05 -0.02 riboflavi… pseudourid…
## 8 VMA13 YPR036W Leucine 0.05 -0.11 vacuolar … hydrogen-t…
## 9 EDC3 YEL015W Leucine 0.05 0.12 deadenyly… molecular …
## 10 VPS5 YOR069W Leucine 0.05 -0.2 protein r… protein tr…
## # ... with 33,168 more rowsNesting versus %>%
So what?
Now, think about this for a minute. What if we wanted to get the correlation between the growth rate and expression separately for each limiting nutrient only for genes in the leucine biosynthesis pathway, and return a sorted list of those correlation coeffients rounded to two digits? Mentally we would do something like this:
- Take the
ydatdataset - then
filter()it for genes in the leucine biosynthesis pathway - then
group_by()the limiting nutrient - then
summarize()to get the correlation (cor()) between rate and expression - then
mutate()to round the result of the above calculation to two significant digits - then
arrange()by the rounded correlation coefficient above
But in code, it gets ugly. First, take the ydat dataset
ydatthen filter() it for genes in the leucine biosynthesis pathway
filter(ydat, bp=="leucine biosynthesis")then group_by() the limiting nutrient
group_by(filter(ydat, bp=="leucine biosynthesis"), nutrient)then summarize() to get the correlation (cor()) between rate and expression
summarize(group_by(filter(ydat, bp == "leucine biosynthesis"), nutrient), r = cor(rate,
expression))then mutate() to round the result of the above calculation to two significant digits
mutate(summarize(group_by(filter(ydat, bp == "leucine biosynthesis"), nutrient),
r = cor(rate, expression)), r = round(r, 2))then arrange() by the rounded correlation coefficient above
arrange(
mutate(
summarize(
group_by(
filter(ydat, bp=="leucine biosynthesis"),
nutrient),
r=cor(rate, expression)),
r=round(r, 2)),
r)## # A tibble: 6 x 2
## nutrient r
## <chr> <dbl>
## 1 Leucine -0.580
## 2 Glucose -0.04
## 3 Ammonia 0.16
## 4 Sulfate 0.33
## 5 Phosphate 0.44
## 6 Uracil 0.580Now compare that with the mental process of what you’re actually trying to accomplish. The way you would do this without pipes is completely inside-out and backwards from the way you express in words and in thought what you want to do. The pipe operator %>% allows you to pass the output data frame from one function to the input data frame to another function.

Nesting functions versus piping
This is how we would do that in code. It’s as simple as replacing the word “then” in words to the symbol %>% in code. (There’s a keyboard shortcut that I’ll use frequently to insert the %>% sequence – you can see what it is by clicking the Tools menu in RStudio, then selecting Keyboard Shortcut Help. On Mac, it’s CMD-SHIFT-M.)
ydat %>%
filter(bp=="leucine biosynthesis") %>%
group_by(nutrient) %>%
summarize(r=cor(rate, expression)) %>%
mutate(r=round(r,2)) %>%
arrange(r)## # A tibble: 6 x 2
## nutrient r
## <chr> <dbl>
## 1 Leucine -0.580
## 2 Glucose -0.04
## 3 Ammonia 0.16
## 4 Sulfate 0.33
## 5 Phosphate 0.44
## 6 Uracil 0.580Piping exercises
EXERCISE 3
Here’s a warm-up round. Try the following.
Show the limiting nutrient and expression values for the gene ADH2 when the growth rate is restricted to 0.05. Hint: 2 pipes: filter and select.
## # A tibble: 6 x 2
## nutrient expression
## <chr> <dbl>
## 1 Glucose 6.28
## 2 Ammonia 0.55
## 3 Phosphate -4.6
## 4 Sulfate -1.18
## 5 Leucine 4.15
## 6 Uracil 0.63What are the four most highly expressed genes when the growth rate is restricted to 0.05 by restricting glucose? Show only the symbol, expression value, and GO terms. Hint: 4 pipes: filter, arrange, head, and select.
## # A tibble: 4 x 4
## symbol expression bp mf
## <chr> <dbl> <chr> <chr>
## 1 ADH2 6.28 fermentation* alcohol dehydrogenase activity
## 2 HSP26 5.86 response to stress* unfolded protein binding
## 3 MLS1 5.64 glyoxylate cycle malate synthase activity
## 4 HXT5 5.56 hexose transport glucose transporter activity*When the growth rate is restricted to 0.05, what is the average expression level across all genes in the “response to stress” biological process, separately for each limiting nutrient? What about genes in the “protein biosynthesis” biological process? Hint: 3 pipes: filter, group_by, summarize.
## # A tibble: 6 x 2
## nutrient meanexp
## <chr> <dbl>
## 1 Ammonia 0.943
## 2 Glucose 0.743
## 3 Leucine 0.811
## 4 Phosphate 0.981
## 5 Sulfate 0.743
## 6 Uracil 0.731## # A tibble: 6 x 2
## nutrient meanexp
## <chr> <dbl>
## 1 Ammonia -1.61
## 2 Glucose -0.691
## 3 Leucine -0.574
## 4 Phosphate -0.750
## 5 Sulfate -0.913
## 6 Uracil -0.880EXERCISE 4
That was easy, right? How about some tougher ones.
First, some review. How do we see the number of distinct values of a variable? Use n_distinct() within a summarize() call.
ydat %>% summarize(n_distinct(mf))## # A tibble: 1 x 1
## `n_distinct(mf)`
## <int>
## 1 1086Which 10 biological process annotations have the most genes associated with them? What about molecular functions? Hint: 4 pipes: group_by, summarize with n_distinct, arrange, head.
## # A tibble: 10 x 2
## bp n
## <chr> <int>
## 1 biological process unknown 269
## 2 protein biosynthesis 182
## 3 protein amino acid phosphorylation* 78
## 4 protein biosynthesis* 73
## 5 cell wall organization and biogenesis* 64
## 6 regulation of transcription from RNA polymerase II promoter* 49
## 7 nuclear mRNA splicing, via spliceosome 47
## 8 DNA repair* 44
## 9 aerobic respiration* 42
## 10 ER to Golgi transport* 42## # A tibble: 10 x 2
## mf n
## <chr> <int>
## 1 molecular function unknown 886
## 2 structural constituent of ribosome 185
## 3 protein binding 107
## 4 RNA binding 63
## 5 protein binding* 53
## 6 DNA binding* 44
## 7 structural molecule activity 43
## 8 GTPase activity 40
## 9 structural constituent of cytoskeleton 39
## 10 transcription factor activity 38How many distinct genes are there where we know what process the gene is involved in but we don’t know what it does? Hint: 3 pipes; filter where bp!="biological process unknown" & mf=="molecular function unknown", and after selecting columns of interest, pipe the output to distinct(). The answer should be 737, and here are a few:
## # A tibble: 737 x 3
## symbol bp mf
## <chr> <chr> <chr>
## 1 SFB2 ER to Golgi transport molecular function…
## 2 EDC3 deadenylylation-independent decapping molecular function…
## 3 PER1 response to unfolded protein* molecular function…
## 4 PEX25 peroxisome organization and biogenesis* molecular function…
## 5 BNI5 cytokinesis* molecular function…
## 6 CSN12 adaptation to pheromone during conjugation … molecular function…
## 7 SEC39 secretory pathway molecular function…
## 8 ABC1 ubiquinone biosynthesis molecular function…
## 9 PRP46 nuclear mRNA splicing, via spliceosome molecular function…
## 10 MAM3 mitochondrion organization and biogenesis* molecular function…
## # ... with 727 more rowsWhen the growth rate is restricted to 0.05 by limiting Glucose, which biological processes are the most upregulated? Show a sorted list with the most upregulated BPs on top, displaying the biological process and the average expression of all genes in that process rounded to two digits. Hint: 5 pipes: filter, group_by, summarize, mutate, arrange.
## # A tibble: 881 x 2
## bp meanexp
## <chr> <dbl>
## 1 fermentation* 6.28
## 2 glyoxylate cycle 5.29
## 3 oxygen and reactive oxygen species metabolism 5.04
## 4 fumarate transport* 5.03
## 5 acetyl-CoA biosynthesis* 4.32
## 6 gluconeogenesis 3.64
## 7 fatty acid beta-oxidation 3.57
## 8 lactate transport 3.48
## 9 carnitine metabolism 3.3
## 10 alcohol metabolism* 3.25
## # ... with 871 more rowsGroup the data by limiting nutrient (primarily) then by biological process. Get the average expression for all genes annotated with each process, separately for each limiting nutrient, where the growth rate is restricted to 0.05. Arrange the result to show the most upregulated processes on top. The initial result will look like the result below. Pipe this output to a View() statement. What’s going on? Why didn’t the arrange() work? Hint: 5 pipes: filter, group_by, summarize, arrange, View.
## # A tibble: 5,257 x 3
## # Groups: nutrient [6]
## nutrient bp meanexp
## <chr> <chr> <dbl>
## 1 Ammonia allantoate transport 6.64
## 2 Ammonia amino acid transport* 6.64
## 3 Phosphate glycerophosphodiester transport 6.64
## 4 Glucose fermentation* 6.28
## 5 Ammonia allantoin transport 5.56
## 6 Glucose glyoxylate cycle 5.28
## 7 Ammonia proline catabolism* 5.14
## 8 Ammonia urea transport 5.14
## 9 Glucose oxygen and reactive oxygen species metabolism 5.04
## 10 Glucose fumarate transport* 5.03
## # ... with 5,247 more rowsLet’s try to further process that result to get only the top three most upregulated biolgocal processes for each limiting nutrient. Google search “dplyr first result within group.” You’ll need a filter(row_number()......) in there somewhere. Hint: 5 pipes: filter, group_by, summarize, arrange, filter(row_number().... Note: dplyr’s pipe syntax used to be %.% before it changed to %>%. So when looking around, you might still see some people use the old syntax. Now if you try to use the old syntax, you’ll get a deprecation warning.
## # A tibble: 18 x 3
## # Groups: nutrient [6]
## nutrient bp meanexp
## <chr> <chr> <dbl>
## 1 Ammonia allantoate transport 6.64
## 2 Ammonia amino acid transport* 6.64
## 3 Phosphate glycerophosphodiester transport 6.64
## 4 Glucose fermentation* 6.28
## 5 Ammonia allantoin transport 5.56
## 6 Glucose glyoxylate cycle 5.28
## 7 Glucose oxygen and reactive oxygen species metabolism 5.04
## 8 Uracil fumarate transport* 4.32
## 9 Phosphate vacuole fusion, non-autophagic 4.20
## 10 Leucine fermentation* 4.15
## 11 Phosphate regulation of cell redox homeostasis* 4.03
## 12 Leucine fumarate transport* 3.72
## 13 Leucine glyoxylate cycle 3.65
## 14 Sulfate protein ubiquitination 3.4
## 15 Sulfate fumarate transport* 3.27
## 16 Uracil pyridoxine metabolism 3.11
## 17 Uracil asparagine catabolism* 3.06
## 18 Sulfate sulfur amino acid metabolism* 2.69There’s a slight problem with the examples above. We’re getting the average expression of all the biological processes separately by each nutrient. But some of these biological processes only have a single gene in them! If we tried to do the same thing to get the correlation between rate and expression, the calculation would work, but we’d get a warning about a standard deviation being zero. The correlation coefficient value that results is NA, i.e., missing. While we’re summarizing the correlation between rate and expression, let’s also show the number of distinct genes within each grouping.
ydat %>%
group_by(nutrient, bp) %>%
summarize(r=cor(rate, expression), ngenes=n_distinct(symbol))## Warning in cor(rate, expression): the standard deviation is zero## # A tibble: 5,286 x 4
## # Groups: nutrient [?]
## nutrient bp r ngenes
## <chr> <chr> <dbl> <int>
## 1 Ammonia 'de novo' IMP biosynthesis* 0.312 8
## 2 Ammonia 'de novo' pyrimidine base biosynthesis -0.0482 3
## 3 Ammonia 'de novo' pyrimidine base biosynthesis* 0.167 4
## 4 Ammonia 35S primary transcript processing 0.508 13
## 5 Ammonia 35S primary transcript processing* 0.424 30
## 6 Ammonia acetate biosynthesis 0.468 1
## 7 Ammonia acetate metabolism 0.929 1
## 8 Ammonia acetate metabolism* -0.686 1
## 9 Ammonia acetyl-CoA biosynthesis -0.851 1
## 10 Ammonia acetyl-CoA biosynthesis from pyruvate 0.0951 1
## # ... with 5,276 more rowsTake the above code and continue to process the result to show only results where the process has at least 5 genes. Add a column corresponding to the absolute value of the correlation coefficient, and show for each nutrient the singular process with the highest correlation between rate and expression, regardless of direction. Hint: 4 more pipes: filter, mutate, arrange, and filter again with row_number()==1. Ignore the warning.
## # A tibble: 6 x 5
## # Groups: nutrient [6]
## nutrient bp r ngenes absr
## <chr> <chr> <dbl> <int> <dbl>
## 1 Glucose telomerase-independent telomere maintenance -0.95 7 0.95
## 2 Ammonia telomerase-independent telomere maintenance -0.91 7 0.91
## 3 Leucine telomerase-independent telomere maintenance -0.9 7 0.9
## 4 Phosphate telomerase-independent telomere maintenance -0.9 7 0.9
## 5 Uracil telomerase-independent telomere maintenance -0.81 7 0.81
## 6 Sulfate translational elongation* 0.79 5 0.79